|
您当前的位置: 气体分离设备商务网 → 行业书库 → 在线书籍 → 《气体爆炸手册(GAS EXPLOSION HANDBOOK )》
Gas Explosions in Process Areas and Unconfined Areas
In Flixborough in 1974, 60 tons of cyclohexane were released inside the process plant. A large dense flammable cloud was formed and when this cloud exploded the plant was totally demolished. The blast from the explosion was estimated to be equivalent to 15 tons of TNT. 29 persons were killed in this accident. In the Port Hudson event, propane was released from an underground pipeline. The release filled up a valley with propane-air mixture. The explosion started as an internal explosion in a pump-house and this triggered the unconfined cloud to detonate. These two cases are examples of large gas explosions in process areas and unconfined situations. Other accidents may be caused by much smaller releases of combustible gas, like the Sarnia incident. In Sarnia (London Free Press, 1984) 10-20 kg of hydrogen leaked out. The cloud detonated and killed two persons. The objective of this chapter is:
11.1 Confinement Figure 11.1 shows an illustration of a process plant explosion where the flame propagates through a fuel-air cloud. If the explosion is a deflagration, as described in Chapter 5, high explosion pressures will only be generated when the gas cloud is inside confined or partly confined areas or engulfs obstructing objects, such as pipework and process equipment. In a process plant the areas where high pressures can be generated by a deflagration, are mainly inside buildings, pipe bridges, in open process area where pipework and process equipment is densely packed and in tunnels and culverts. However, if the cloud detonates as a result of a strong flame acceleration, the detonation will be able to propagate through the cloud without any confinement or obstructing objects (see Chapter 6).
11.2 Fuel When a fuel is accidentally released, the density of the fuel is an important parameter for the formation of the combustible cloud. When the gas is lighter than air, like hydrogen, buoyancy will make the cloud rise. In an open situation, the gas will rise and be dispersed relatively quickly. A dense gas will drift along the ground, and will not disperse as fast as a light gas. The dense gas may drift into buildings, tunnels, culverts or other confined areas. A release of a dense gas therefore has a higher potential of forming larger fuel air clouds than a release of a light gas. In Chapters 5 and 6 we discussed flame acceleration and detonability of different types of fuels. For otherwise equal conditions, the different fuels mixed with air will generate different explosion pressures. Figure 11.2 shows some experimental results with different fuel-air mixtures in a specific apparatus. Even though the pressure will be different in other situations, the relative fuel ranking, as shown in Figure 11.2, seems to constitute a general trend. In an accidental situation, we can therefore expect that hydrogen and ethylene will give higher explosion pressures than fuels like propane and methane for the same size of gas cloud and with other conditions being similar as well.
Hydrogen is a fuel that is lighter than air, disperses relatively fast, and causes high explosion pressures. If we review loss experience with hydrogen, we will find that the sizes of the explosions are fairly limited. The larger hydrogen explosions are typically equivalent to a few hundred kg of TNT, which is significantly less than accidents like Flixborough, which was equivalent to 15 tons of TNT. Even though the size of the hydrogen explosion is limited, the local damage in the area where the explosion takes place is very severe. Hydrogen is very reactive and a deflagration may accelerate very fast and easily transit into a detonation. Several accidents have been reported where hydrogen clouds are likely to have detonated. Sarnia was definitely a detonation in a free hydrogen cloud. In records from accidental releases of heavier-than-air fuels, you will find large varieties of accidents. In this section we have discussed fuel type as an important parameter characterising the consequences of a gas explosion. 11.3 Flash Fires The term "flash fire" is often used for a deflagration producing negligible overpressure. Various large scale tests (Lind and Whitson, 1977; Hirst and Eyre, 1983; Zeeuwen et al., 1983; Harrison and Eyre, 1987a) have demonstrated that a truly unconfined, unobstructed gas cloud ignited by a weak ignition source will produce only small overpressures while burning. There are no mechanisms that can accelerate the flame (i.e. a deflagration) to more than a few tens of meters per second under these conditions. The combustion is so slow that burned gas will expand before any significant pressure can build up. The thermal effect is the main hazard from a truly unconfined deflagrating cloud. However, if the same free cloud detonates due to transition to detonation in a confined neighbouring area, the result will be a very strong blast wave. Detonations are discussed in Chapter 6. When a fuel-air cloud burns, the hot combustion products will rise due to buoyancy. For a large cloud this buoyancy can be very strong and the flow ahead of the flame can even be reversed, as indicated in Figure 11.3. In the accident in Ufa in 1988 where a train ignited a large gas cloud from an LPG pipeline, the wind forces caused by the buoyancy were so strong that the trees tilted. (Borisov, 1989.) This Ufa event is an extreme case, since the cloud was extremely large.
11.4 Buildings and other Partly Confined Areas In the previous chapter gas explosions in compartments and offshore modules were discussed. The information in Chapter 10 is directly relevant for evaluation of explosions in buildings in process plants. In a process plant combustible gas may be formed as a result of a leak inside the building or gas drifting into the building. The consequences of a gas explosion inside the building will mainly depend on the type of fuel, size and concentration of the gas cloud, ignition and geometrical layout, i.e. confinement, venting and obstructing objects. In a building with no or little explosion venting, the building will confine the explosion and high explosion pressures may be generated. Vent openings are of major importance in keeping the explosion pressure down. In the period 1965-1975 there were a large number of gas explosions in buildings, particularly in compressor buildings (Kletz, 1987). One reason for this large number of explosions was the design of the buildings. Due to weather conditions as well as noise the buildings were closed. In a closed building a release of a few kilograms of fuel can cause a serious explosion. Even with forced ventilation, a flammable gas cloud can easily be generated. Again referring to Trevor Kletz (1987) is pertinent: "The best building has no walls". 11.5 Pipe Bridges In a process plant a pipe bridge can be fairly congested and can therefore support flame acceleration and cause high explosion pressures. Figure 11.4 illustrates flame propagation through a pipe bridge (Bjerketvedt and Nornes, 1989). In the FLACS simulation the fuel-air cloud was ignited at ground level. The flame was therefore propagating in a spherical mode until it reached the pipe bridge. In the pipe bridge, the flow ahead of the flame was turbulent and the flame was therefore accelerating. In Figure 11.4 we can see that the flame has propagated a longer distance at a higher velocity in the pipe bridge than at ground level. In the numerical simulation, the explosion pressure was predicted to be approx. 200 mbar. This value was of course only valid for this particular geometry. In British Gas experiments, in a 1:5 scale pipe bridge geometry with propane-air, even transition to detonation was observed when the ignition source was a strong jet flame.
11.6 Open Process Areas An open process area can also be very congested. Pipework, process equipment, tanks etc. will contribute to turbulence generation during an explosion. The experimental results presented in section 5.5 (from CMR's cubical vessel), show that a spherical gas explosion in a very obstructed area only needs a few meters of flame travel before the explosion pressure reaches levels that can cause severe damage. To avoid damaging overpressures it is therefore important to keep congestion to a minimum and not make congested areas too large. It should be noted that extensive obstructedness also may act as confinement! Tanks and process vessels should not be located too close to each other. Figure 11.5 shows a row of tanks. During a gas explosion, the flame will propagate under the tanks and the tanks will act as repeated obstacles and accelerate the flame. (See Figure 5.7). The venting area is, in this case, mainly dependent on the space between the tanks and their length. The longer the spacing, the better is the venting. To avoid strong flame acceleration it is therefore important to ensure that satisfactory equipment spacing exists. Van Wingerden and Zeeuwen (1986) have performed tests in relevant geometries, the results of which support this statement. The optimum equipment spacing is scenario dependent and can be estimated by performing gas explosion simulations with FLACS or µFlacs.
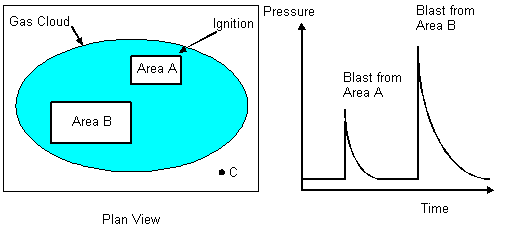
11.7 Tunnels and Culverts In an accidental release in a process area or an open area, dense gas clouds have a tendency to flow into underground systems like sewers, culverts, tunnels etc. If a gas cloud manages to enter such areas and ignite, the explosion will be an internal explosion, as discussed in Chapter 9. Another event (or phenomenon) that can cause high explosion pressures and possibly transition to detonation (Moen et al. 1989) is jet ignition. Figure 11.1 shows an example of jet ignition of an unconfined cloud, due to a confined explosion in a sewer system. Such a jet flame shooting out from a confined region is a very strong ignition source that may cause high pressure explosions. 11.8 Multiple Explosions and Blast Waves A large release may form a large explosive cloud that may cover many confined and/or congested areas. We have illustrated the situation in Figure 11.6. If we assume ignition close to Area A, we will get a flame acceleration, i.e. explosion within this area. If the explosion does not transit into a detonation in Area A, the flame speed will decay as it propagates into the open area between Areas A and B. Results from experiments showing deceleration on flame exiting from an area containing repeated obstacles into an unobstructed area are presented in Figure 5.11. In the open area, between Areas A and B, the flame may propagate at a few tens of meters per second. The time for propagation from Area A to Area B will be fairly long compared to the time to burn the clouds within these areas. When the flame reaches Area B there may be a new explosion. At the location C we may get two blast waves as shown in the figure. This shows that one gas cloud may cause several local explosions.
The effect of localised explosions may in some situations not only cause high pressures locally but also cause high velocity flames to propagate into less confined but obstructed regions, where the high velocity of the flame may be sustained. Some recently published data by Harris and Wickens (1989) show examples of such an effect which was observed when flame propagation in repeated obstacle arrays was studied. They showed that if a flame entered the unconfined obstacle array at a high velocity, the flame was able to stabilise at a high velocity and high explosion pressure. However, if the flame had a low velocity in the beginning of the array, it was not able to accelerate to high velocities and the corresponding explosion pressure was low. 11.9 FLACS Simulations The FLACS code was originally developed for simulation of gas explosions in offshore modules. In a process plant a gas explosion scenario will involve a larger variety of parameters than for an offshore module. In a process plant the fuel may be very reactive like hydrogen and ethylene, it may be a mixture of fuels or any kind of single fuel. The geometrical layout will also vary greatly from case to case. The physics of gas explosions in a process plant is of course the same as in an offshore module. The models in FLACS should be capable of handling gas explosions also in process plants. Bjerketvedt and Nornes (1989), Savvides and Tam (1991) and Salvesen and van Wingerden (1993) have used the FLACS code for simulation of gas explosions in process plants. The simulations performed by Salvesen and van Wingerden (1993) considered a large process plant in Beek in The Netherlands which was involved in a strong vapour cloud explosion in 1975. The process plant involved was a naphtha cracker (Naphtha Cracker II) plant (dimensions: 160 m × 70 m × 40 m). After a major release of what according to the official report must have been C3-C4 hydrocarbons (propylene, butane) ignition occurred resulting in the death of 14 people, 107 injured people and extensive damage to the plant. The investigation after the incident revealed explosion pressures based on calculations on the damage (up to 1 bar locally), the size and location of the flammable cloud and the point of ignition. The simulations revealed that the gas that was involved in the accident was not propylene as assumed by some of the sources used in the official report but more likely ethylene, or a mixture of ethylene and propylene or butadiene. Pressures generated for propylene were in the order of 14-15 mbar, whereas for ethylene pressure in the order of 10-14 bar were generated. Unknown factors such as mixture composition and concentration profile in the cloud make it more or less impossible to simulate the explosion in detail. Nevertheless the simulations showed the possibilities of FLACS also for process plants. Figure 11.7 shows the plant as it was represented by FLACS.
11.10 Plant Layout To keep the loss potential low, it is important to separate different units and buildings. Different process areas should be kept separated in order to avoid domino effects. Keeping congestion to a minimum is also important. All activities not absolutely necessary for the operation of the plant should be placed away from potentially hazardous areas. Buildings which may be subjected to blast from explosions, should be blast resistant. Figure 11.8 shows an example of restrictions on design and layout of a process plant, as suggested by Kletz (1988).
11.11 Guidelines
注意:本栏目所有书籍均来至于网络,如果其中涉及了您的版权,请及时联系我们,我们在核实后将在第一时间予以删除!
|